|
| Systemically delivered Photoreceptor Progenitor cells reversed the progression of photoreceptor degeneration – and promoted regeneration of both Rods and Cones.[4] |
Author: Irv Arons
Reversing Retinal Cell Death With Inkjet Printing?
 |
|
Inkjet Setup
|
 |
| Figure 1. Retinal Cell Printing. Image sequences of (a) retinal cells and (b) purifed glial cells as they are ejected from the nozzle, labeled with image capture time (1). |
 |
| Barbara Lorber |
 |
| Keith Martin |
Laser Refractive Keratoplasty: The Rest of the Story
The article will be part of a book that the OSA is publishing in 2016 to celebrate its 100th Anniversary. The book will capture the history of the Optical Society in the context of the evolution of optics research and the optics industry as well as changes in the nature of the science and technology enterprise and, even more broadly, changes in the United States and the world.
In reading the article, written by Dr. James Wynne, the manager of the IBM’s Watson Research Center laboratory, where the research took place, I realized that it was the first part of the story that I had told in my White Paper, noted above. I also realized that I had a connection with Dr. Wynne, who it turns out is a relative of a close friend of mine. So, I reached out to Dr. Wynne to get his approval to use excerpts from his article to provide the beginnings of how laser refractive surgery, using the excimer laser, really began, i.e., the “rest of the story”.
So, here in Dr. Wynne’s own words, is how the excimer laser was first used in ablating human tissue and became the device to use in performing PRK (surface ablation of the cornea, including the epithelium), at first, and then LASIK (mechanical or femtosecond laser formation of an epithelium flap followed by ablation of the corneal stromal surface) today.
Excimer Laser Surgery – Laying the Foundation for Laser Refractive Surgery
James J. Wynne, Ph. D.
The discovery of excimer laser surgery
On November 27, 1981, the day after Thanksgiving, Dr. Rangaswamy Srinivasan brought leftovers from his Thanksgiving dinner into the IBM Thomas J. Watson Research Center, where he irradiated turkey cartilage with ~10-nsec pulses of light from an argon fluoride (ArF) excimer laser. This irradiation produced a clean-looking “incision” in the cartilage, as observed through an optical microscope. Subsequently, Srinivasan and his IBM colleague, Dr. Samuel E. Blum, team carried out further irradiation of turkey cartilage samples under controlled conditions, measuring the laser fluence and the number of pulses used to produce the incisions. Srinivasan gave a sample to me, and, for comparison, I irradiated it with ~10-nsec pulses of 532-nm light from a Q-switched, frequency-doubled, Nd:YAG laser. This irradiation did not incise the sample; rather it created a burned, charred region of tissue.
Srinivasan, Blum and I realized that we had discovered something novel and unexpected, and we wrote an invention disclosure, completed on Dec. 31, 1981. Our disclosure described multiple potential surgical applications, on hard tissue (bones and teeth) as well as soft tissue. We anticipated that the absence of collateral damage to the tissue underlying and adjacent to the incision produced in vitro would result in minimal collateral damage when the technique was applied in vivo. The ensuing healing would not produce scar tissue. We recognized that we had a laser surgical method that was a radical departure from all other laser surgical techniques that had been developed since the operation of the first laser on May 16, 1960. Rather than photocoagulating the irradiated tissue, the excimer laser was ablating a thin layer of tissue from the surface with each pulse, leaving negligible energy behind, insufficient to thermally damage the tissue underlying and adjacent to the incised volume. This insight was unprecedented and underlies the subsequent application of our discovery to laser refractive surgery.
Background to this discovery
Since 1976, as manager of the Laser Physics and Chemistry department of IBM’s T. J. Watson Research Center, one of my responsibilities was to ensure that we had access to the best and latest laser instrumentation. Earlier, I had used a nitrogen laser, emitting short pulses of ultraviolet light at 337-nm, to pump fluorescent dyes that emitted visible and near infrared light, which he used for laser spectroscopic studies. When the excimer laser, a higher-power, pulsed source of ultraviolet radiation became commercially available, I purchased a unit for use by the scientists in my department. Srinivasan had devoted his entire research career since 1960 to study the action of ultraviolet radiation on organic materials, e.g., polymers. In 1980, he and his technical assistant, Veronica Mayne-Banton, discovered that the ~10-nsec pulses of far ultraviolet radiation from the excimer laser could photoetch solid organic polymers, if the fluence of the radiation exceeded an ablation threshold.
Since organic polymers proved susceptible to etching by the excimer laser irradiation, we reasoned that an animal’s structural protein, such as collagen, which contains the peptide bond as the repeating unit along the chain, would also respond to the ultraviolet laser pulses. We knew that when skin was incised with a sharp blade, the wound would heal without fibrosis and, hence, no scar tissue. Conceivably, living skin or other tissue, when incised by irradiation from a pulsed ultraviolet light source, would also heal without fibrosis and scarring.
Next steps
To develop practical innovative applications from our discovery, it was clear that we had to collaborate with medical/surgical professionals. In order to interest these professionals, we etched a single human hair by a succession of 193-nm ArF excimer laser pulses, producing a SEM micrograph (Fig. 1), showing 50-ƒÝ-wide laser-etched notches.
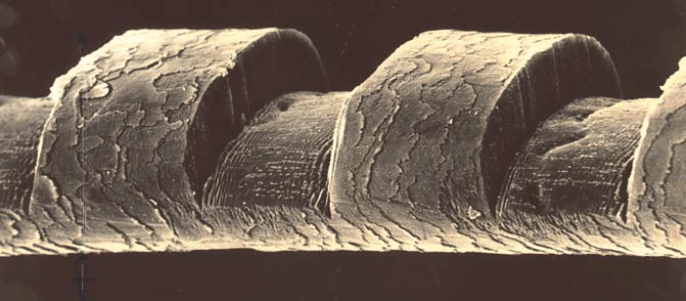 |
| Fig. 1 – Scanning electron micrograph of a human hair etched by irradiation with an ArF excimer laser; the notches are 50 ƒÝ wide. |
While IBM Intellectual Property Law was preparing a patent application, we were constrained from discussing our discovery with people outside IBM. But we had a newly hired IBM colleague, Ralph Linsker, with an M.D. and a Ph. D. in physics. Linsker obtained fresh arterial tissue from a cadaver, and we irradiated a segment of aorta with both 193-nm light from the ArF excimer laser and 532-nm light from the Q-switched, frequency-doubled Nd:YAG laser. Once again, the morphology of the tissue adjacent to the irradiated/incised regions, examined by standard tissue pathology techniques, was stunningly different, with irradiation by the 193-nm light showing no evidence of thermal damage to the underlying and adjacent tissue.
This experimental study on freshly excised human tissue confirmed that excimer laser surgery removed tissue by a fundamentally new process. Our vision–that excimer laser surgery would allow tissue to be incised so cleanly that subsequent healing would not produce scar tissue–was more than plausible, it was likely, subject to experimental verification on live animals.
First public disclosure
After IBM filed our patent application on December 9, 1982, we were authorized to publicly disclose our discovery. We wrote a paper and submitted it to Science magazine, but it was rejected, because one of the referees argued that the irradiation of living humans and animals with far ultraviolet radiation would be carcinogenic, making our laser surgical technique more harmful than beneficial. Since Srinivasan now had an invitation to give a presentation about his work on polymers at the upcoming CLEO 1983 conference in Baltimore, MD, co-sponsored by the Optical Society of America, we wanted to get a publication into print as soon as possible, so we resubmitted our paper to the trade journal Laser Focus, including some remarks about the new experiments on human aorta. Serendipitously, the Laser Focus issue containing our paper was published simultaneously with CLEO 1983, where, on May 20, Srinivasan gave an invited talk entitled “Ablative photodecomposition of organic polymer films by far-UV excimer laser radiation.” In this talk, he gave the first oral public disclosure that the excimer laser cleanly ablated biological specimens as well as organic polymers.
From excimer laser surgery to ArF excimer laser-based refractive surgery
At this very same CLEO 1983 meeting, on May 18, Stephen Trokel and Francis L’Esperance, two renowned ophthalmologists, gave invited talks on applications of infrared lasers to ophthalmic surgery. I attended both of their talks and was amazed at the results they obtained in successfully treating two very different ophthalmic conditions. I was well aware that the ruby laser was first used to eradicate a retinal lesion in late 1961, and retinal surgery with lasers had become widespread in the ensuing two decades, in particular to repair retinal tears and to treat diabetic retinopathy. But these treatments required a laser at a wavelength for which the ocular media anterior to the retina was transparent. Excimer laser light would be absorbed in a thin layer upon entering the cornea, so the excimer laser would be useless for treating retinal maladies.
But Trokel knew of ophthalmic conditions, such as the refractive imperfection known as myopia, that could be corrected by modifying the corneal curvature. A treatment known as radial keratotomy (RK), developed in the Soviet Union and being practiced in the United States, corrected myopia by using a cold steel scalpel to make radial incisions at the periphery of the cornea. When these incisions healed, the curvature of the front surface of the cornea was reduced, with the consequence that the patient’s myopia was also reduced. The technique could rarely give the patient uncorrected visual acuity of 20/20, but the patient’s myopia was definitely reduced. One serious drawback of RK was that the depth of the radial incisions left the cornea mechanically less robust, and the healed eye was more susceptible to “fracture” under impact, such as might occur during an automobile collision. Trokel speculated that the excimer laser might be a better scalpel for creating the RK incisions.
Upon learning of our discover of excimer laser surgery, Trokel, who was affiliated with Columbia University’s Harkness Eye Center in New York City, contacted Srinivasan and subsequently brought enucleated calf eyes (derived from slaughter) to our IBM Research Center on July 20, 1983. Srinivasan’s technical assistant, Bodil Braren, participated in an experiment using the ArF excimer laser to precisely etch the corneal epithelial layer and stroma of these calf eyes. The published report of this study is routinely referred to by the ophthalmic community as the seminal paper in laser refractive surgery.
To conduct studies on live animals, the experiments were moved to Columbia’s laboratories. Such experiments were necessary to convince the medical community that living cornea etched by the ArF excimer laser does not form scar tissue at the newly created surface and the etched volume is not filled in by new growth. The first experiment on a live rabbit in November, 1983, showed excellent results in that, after a week of observation, the cornea was not only free from any scar tissue, but the depression had not filled in. Further histological examination of the etched surface at high magnification showed an interface free from detectable damage.
L’Esperance, also affiliated with Columbia’s Harkness Eye Center, thought beyond RK and, in November, 1983, filed a patent application describing the use of excimer laser ablation to modify the curvature of the cornea by selectively removing tissue from the front surface, not the periphery, of the cornea. His U.S. Patent No 4,665,913 specifically describes this process, which was later named photorefractive keratectomy (PRK).
Soon ophthalmologist around the world, who knew of the remarkable healing properties of the cornea, were at work exploring different ways to use to excimer laser to reshape the cornea. From live animal experiments, they moved to enucleated human eyes, then to blind eyes of volunteers, where they could study the healing. Finally, in 1988, a sighted human was treated with PRK (Editors Note: by Dr. Marguerite McDonald at LSU), and after the cornea had healed by epithelialization, this patient’s myopia was corrected.
To read the complete article written by Dr. Wynne, including his footnotes, please follow this link.
AMD Update 25: Results of The AREDS2 HOME Study of Notal Vision’s Home Monitoring Device for AMD Announced
AMD Update 24: DARPins Phase 2 Trial Results Fall Short
Gene Therapy in Ophthalmology Update 21: New Gene Therapy Company, Spark Therapeutics, Launches
 |
|
| Corey Haas, his parents, and the CHOP team that treated his Leber’s and gave him back his vision. |
 |
| Read Corey’s story in Ricki Lewis’ book, The Forever Fix: Gene Therapy and the Boy Who Saved It. |
Gene Therapy in Ophthalmology Update 20: Oxford BioMedica Clinical Trials Resume
Research in Retinal Disease: The Foundation Fighting Blindness Invests $2.1 Million in Seven New Research Efforts
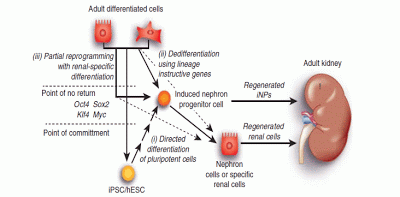
A New Approach to Treating Chronic Kidney Disease: Replenishing Your Nephrons
Gene Therapy in Ophthalmology Update 19: A New Virus Vector for Safer Delivery of Gene Therapies
Stem Cells in Ophthalmology Update 25: ACT Patient in Dry AMD Trial Goes from 20/400 to 20/40!
A New Technique for Restoring Normal Vision to the Blind: The Technology of Prof. Sheila Nirenberg of Weill Cornell Medical School
 |
|
Fig. 1. The transformations of images into patterns of action potentials by the retina.
|
Recently Published Articles: Current Status of Stem Cells and Gene Therapy in Ophthalmology
A Personal Journey: How I Went From Being A Bench Chemist to An Expert Resource in Ophthalmology and Medical Lasers
People in the NorthWest with X-linked retinoschisis
XLRS Natural History Study Beginning in Portland, Oregon
Knowledge gained from the XLRS natural history study will aid in the design of an XLRS gene therapy clinical trial slated to begin in late 2014 or early 2015. The trial will be a collaboration between Applied Genetic Technologies Corporation, OHSU and the Foundation.
X-linked retinoschisis occurs almost exclusively in males. Participants in the natural history study must be of that gender. Otherwise, to qualify, they must:
- have a clinical diagnosis of XLRS
- have a disease-causing mutation in the gene RS1
- be 7 years of age or older
- be able to provide consent/assent (understand study procedures and risks)
Gene Therapy in Ophthalmology Update 18: A RetroSense Update
Gene Therapy in Ophthalmology Update 17: Hemera Biosciences Obtains Initial Funding
Oraya IRay Update 2: INTREPID Two-year Results Meet Primary Clinical Endpoint – Results in At Least 35% Fewer anti-VEGF Injections — Oraya Joins with Optegra to Provide Treatments in the UK
AMD Update 23: DARPins, The Next “Game Changer” for Wet AMD?
Gene Therapy in Ophthalmology Update 16: Current Tables Now Online
I finally figured out how to put my current gene therapy tables online for anyone interested to access. So, here is a brief description of what is available and how to access them:Gene TherapyGene Therapy Companies/Institutions Active in OphthalmologyT…